

Paradigm Shift Intervention Monitoring
 twitter
twitter

Commentary
Recent Seasonal
H3N2 Cases Raise Pandemic Concerns
Recombinomics
Commentary 22:30
August 31, 2012
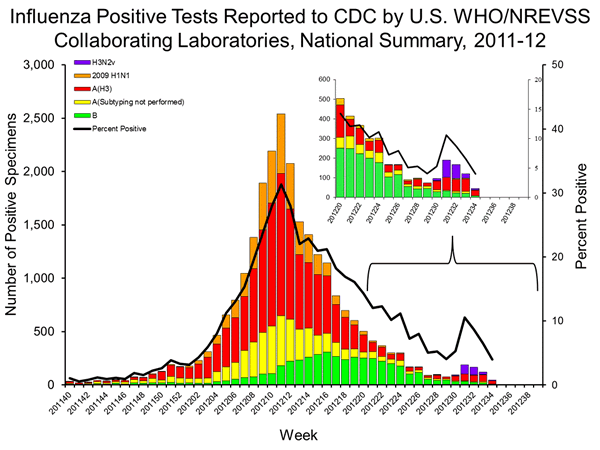
Both seasonal H3N2 and H3N2v samples test as a positive for H3. H3N2v also returns a positive for the H1N1pdm09 NP target. However, H3N2v samples with low RNA levels can generate a positive H3 and a false negative NP and be misdiagnosed as seasonal H3N2.
However, these samples can be conclusively characterized by sequencing, which is done by the CDC, which has been overwhelmed by samples with swine exposure. Therefore, it is far from clear that these recent seasonal H3N2 samples have been tested beyond the state labs, which use the CDC RT-PCR kit to sub-type these samples.
The sequence data of the swine exposure cases identifies a novel H3N2v that has clonally expanded across the country, while the USDA data has failed to identify this novel sample in collections prior to the appearance of this sub-clade at a West Virginia day care center in late 2011, and published swine sequences only have two matches. In contrast, the USDA has identified 26 matches with the H3N2v identified in human cases preceding the novel sub-clade in West Virginia, yet all 2012 human sequences match the West Virginia sub-clade and do not match the earlier 2011 human cases.
This discordance strongly supports clonal expansion in humans, not swine.
Therefore, the 188 “seasonal” H3N2 case listed for weeks 31-33 should be sequenced by the CDC immediately, with priority given to samples from children or those from Kentucky, West Virginia, Ohio, and Indiana.
Recombinomics
Presentations
Recombinomics
Publications
Recombinomics
Paper
at Nature Precedings